Main function:
Filtration sterilization in LVP, SVP, beverage & wines, antibiotic and pharmaceutical water.
| Outer diameter | 68mm |
| Inner diameter | 33mm |
| Length (inch) | 5, 10, 20, 30, 40 |
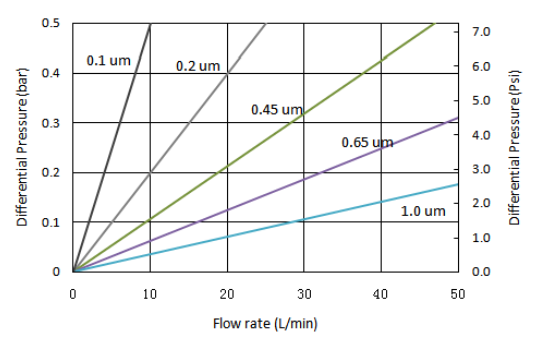
| Removal ratings (µm) | 0.1, 0.22, 0.45, 0.65, 1.0 |
| Effective filter area | ≥0.65㎡/10in |
| Maximum operating temperature | 80 ˚C / 176 ˚F |
| Maximum forward differential pressure | 0.4MPa @ 23 ˚C /58.8 psid @ 73.4 ˚F |
| Steam sterilisation | 121℃/30min |
| Materials of construction | Materials |
| Membrane | Nylon |
| Support layers | Polypropylene |
| Inner Core | Polypropylene/Stainless steel |
| Outer core | Polypropylene/Polyethylene mesh |
| End Cap | Polypropylene |
| O-ring | Silicone/Viton/EPDM/PEFE |
Quality Control
100% integrity testing before package.
Marked with unique serial number for identification and traceability
Endotoxin level <0.25 EU/ml
All components meet with FDA food additive as per 21 CFR 177-182
Manufactured under ISO 9001:2015 certified quality management system and GMP
Nylon Membrane Filter Cartridges are disinfecting grade cartridges that feature a nylon media. This material is well-suited for the filtration of solvents due to its broad affinity and low extractables. Additionally, due to the particles present in water and other liquids having a net negative charge, they can be effectively captured by the positively charged nylon membrane. The filter cartridges are equipped with a heavy duty molded cage, ensuring they can withstand high pressure drops. Furthermore, they are characterized by a hydrophilic Nylon membrane that provides high throughputs, low extractables, and good mechanical strength; they are regularly used for sterile filtration in Active Pharmaceutical Ingredients production.
1. Extractability is low, leading to low pressure drop and enhanced absorption.
2. It is compatible with many chemicals.
3. Throughputs are high, making it cost-efficient and it has a longer lifespan.
4. It can be sterilized using steam.
5. Positive zeta potential effectively removes residual toxins.
1. Parenteral Solutions (SVP, LVP), Reagents, and Buffers.
2. Vaccines, Sera, and Blood Fractions.
3. Particulate and Contamination Control.
4. Cell Culture Fluids.
5. High-Purity Deionized Water and Water for Injection Systems.