Main function: Filtration sterilization in biopharmaceuticals and food & beverage industry.
| Materials of construction | Materials |
| Membrane | Single layer, or double layer asymmetric PES |
| Support layers | Polypropylene |
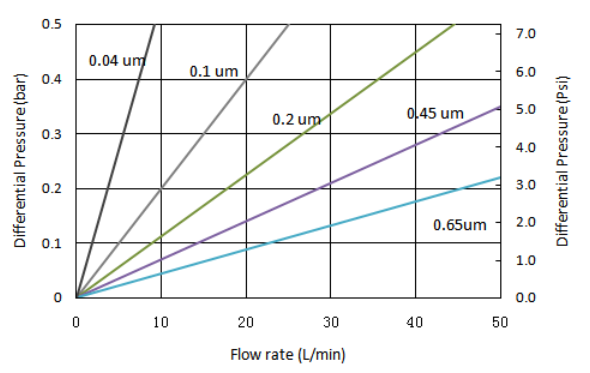
| Removal ratings (µm) | 0.1, 0.22, 0.45, 1.0 |
| Effective filter area | ≥0.65㎡/10in |
| Maximum operating temperature | 80 ˚C / 176 ˚F |
| Maximum forward differential pressure | 0.4MPa @ 23 ˚C /58.8 psid @ 73.4 ˚F |
| Steam sterilisation | 121℃/30min |
Quality control
100% integrity testing before package.
Marked with unique serial number for identification and traceability
Endotoxin level <0.25 EU/ml
All components meet with FDA food additive as per 21 CFR 177-182
Manufactured under ISO 9001:2015 certified quality management system and GMP
PES Cartridges are manufactured with Polyethersulfone to guarantee uniform pore distribution, allowing for maximum bacteria retention. The upstream and downstream polyester support renders it completely resistant to pressure shocks. Being hydrophilic, the Polyethersulfone membrane facilitates easy integrity testing when daily controls are necessary. It is compatible with a broad range of chemicals and does not contain surfactants, offering an absolute rating for the most stringent filtration applications. It is commonly employed for sterile filtration in the production of Active Pharmaceutical Ingredients.
1. Outstanding hydrolysis durability and chemical compatibility.
2. Unique pore size distribution providing full bacterial filtering.
3. End caps and connectors are sealed by heat treatment without any adhesive.
4. Low pressure drop and high flow velocity.
5. Precisely rated.
6. Can be sterilized using autoclave or in situ steam sterilization.
7. 100% integrity test is available.
1. LVP and SVP sterilization filtration processes.
2. Biological products requiring sterilization filtration.
3. Beverage and wine needing sterilization filtration.
4. Freeze‐dried powder necessitating sterilization filtration.
5. Pure water and mineral water require sterilization filtration.
6. High purity chemical reagent requiring sterilization filtration.